Abstract
Introduction: Treatment related toxicities are a major contributor to morbidity during induction therapy in childhood ALL. Our prior study in 103 children with ALL suggested that low intakes of antioxidant micronutrients were associated with toxicity and delays in chemotherapy administration (Am J Clin Nutr 79:1029, 2004). The objective of this study was to examine whether inadequate antioxidant micronutrient dietary intake in children with ALL is associated with episodes of infection or ≥grade 3 mucositis during Remission Induction.
Methods: Assessment of dietary intake was collected in patients (pts) ages 1-17.9 years enrolled on the DFCI ALL Consortium Protocol 05-001 from 2005-2011. Institutional review board approval was obtained by each of the 9 participating centers. Grade 3 or higher (NCI CTCAEvs) mucositis, culture positive bacteremia and fungemia, and radiographic invasive fungal disease were prospectively collected. Dietary intake was assessed with the Harvard Service Food Frequency Questionnaire (FFQ) for children ages 1-5 years and the Youth and Adolescent Harvard FFQ for children ages 5-18 years and queried about intake in the past 30 days at the end of induction. Dietary antioxidant intake from food sources ± supplementation was examined by comparing consumption above and below recommended intake for age and sex as per the Dietary Reference Intake (DRI). Micronutrient intake was summarized with descriptive statistics and plotted by the presence/absence of a given toxicity in induction. Categorical and continuous variables were compared between group with the Fisher's exact test and the Wilcoxon rank sum test respectively. Logistic regression was used to model the probability of a given toxicity univariately and with a multivariable adjustment for sex and age.
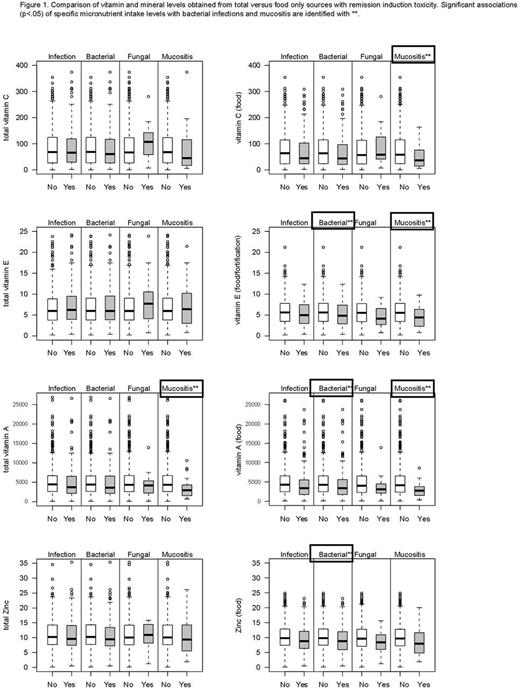
Results: Among 794 eligible pts, 561 (71%) had analyzable FFQs. The sample with dietary surveys responses was marginally younger (p=0.06) and more often classified as initial standard risk (p=0.01) than those with no end of induction survey. DRI recommendations were not met for vitamin C (18%), vitamin E (67%) and zinc (12%); 39% of pts reported zinc overconsumption. 141 (25%) of pts with dietary data had ≥1 bacterial and/or fungal infection, 131 (23%) had ≥1 bacterial infection, and 19 (3%) had ≥1 fungal infection. 24 (4%) pts experienced at least 1 event of ≥grade 3 mucositis. Female sex was associated with overall and bacterial infection (p=0.02) and marginally with mucositis (p=0.06). The proportion of patients with ≥Grade 3 mucositis was marginally higher (p=0.06) for those not meeting the vitamin C recommendation (OR=2.3; 95% CI 1.0-5.5), but was not significant (p=0.10) in a multivariable model. Supplementation was reported by 88 (16%) for zinc, vitamins C, E, and A in combination, 16 (3%) for β-carotene and total carotene, and 87 (15%) for vitamin E alone. Intake levels of vitamins C, E, and A, zinc and total carotenoids from food sources and total (food + supplementation) were compared for pts with and without infection and mucositis (unadjusted for age/sex) (Figure 1). Bacterial infection was associated with low intake from food only of zinc (p=0.02), vitamin A (p=0.01), and vitamin E (p=0.049). Mucositis was associated with the following: low intakes of vitamin C from food (p=0.04), vitamin E from food (p=0.03), total lycopene (p=0.03), total α-carotene (p=0.01), total β-carotene (p=0.007) and from food (p=0.008), total carotene (p=0.005) and carotene from food (p=0.006), and total vitamin A (p=0.01) and vitamin A from food (p=0.004). Females age <10 had higher rates of bacterial infection with low β-carotene (p=0.05) and vitamin A from food (p=0.004). In males age ≥10, low vitamin E from food was observed in those with bacterial infections (p=0.05). Supplementation did not confer an added benefit for most nutrients.
Conclusions: Confirming our pilot study, data from a large prospective cohort show that low dietary intakes of antioxidant vitamins, carotenoids and zinc primarily from food sources predict risk for bacterial infection or mucositis during induction therapy for childhood ALL. Dietary supplementation for most vitamins and minerals did not confer an added benefit for prevention of infection or mucositis over levels obtained from food. Our results suggest a possible modifiable dietary intervention to reduce toxicity during induction therapy for ALL.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal